Downloads
Laboratory Glass
Properties of manufactured glass
Glass tubes made from two types of glass are utilized for the production of laboratory volumetric glassware and other types of laboratory glassware that are in the production program of the Company.
Excellent physical and chemical properties of this type of glass enable using of this glass not only for production of laboratory glassware but for various others purposes, as for instance in the pharmacy (vials), medicine (glass syringes), at production of brilliant lighting unit, at various branches of the textile industry (fingers) and in other further ranges where is required higher temperature and chemical resistance.
Soda-lime glass
This glass has good chemical as well as physical properties and it is suitable for the products that resist momentarily the impact of chemical medium and limitedly also temperature differences. Both types of pipettes are produced from the soda-lime glass.
Chemical composition of manufactured glass
| Oxide |
Borosilicate glass
|
Soda-lime glass
|
Neutral glass
|
|---|---|---|---|
| SiO2 |
80,6 % by weight
|
70 % by weight
|
68 % by weight
|
| Na 2 O + K 2 O |
4 % by weight
|
14 % by weight
|
17 % by weight
|
| Al 2 03 |
2,4 % by weight
|
4 % by weight
|
0,6 % by weight
|
| B 2 03 |
13 % by weight
|
1 % by weight
|
0,1 % by weight
|
| CaO + MgO |
-
|
7 % by weight
|
6 % by weight
|
| BaO |
-
|
3 % by weight
|
4 % by weight
|
Physical properties of manufactured glass
|
Borosilicate glass
|
Soda-lime glass
|
Neutral glass
|
|
| Coefficient of linear thermal expansion (ISO 7991) α (20 °C;300 °C) |
3,3.10-6 K-1
|
8,6.10-6 K-1
|
9,7.10-6 K-1
|
| Density ρ (20 °C) |
2,23 g cm-3
|
2,52 g cm-3
|
2,585 g cm-3
|
| Upper annealing temperature (ISO 7884-7) - viscosity η 1013,2 dPa × s |
560 °C
|
520 °C
|
520 °C
|
| Lower annealing temperature (ISO 7884-7) - viscosity η 1014,7 dPa × s |
510 °C
|
-
|
480 °C
|
Thermally treated glass is annealed, it means tempered up to the upper annealing temperature. It is kept at this temperature for at least 30 minutes and then it is slowly cooled down to the lower annealing temperature. The annealing below this temperature could be even faster. The procedure ensures removal of tension inside the glass that is usually created during its thermal treatment.
Chemical properties of manufactured glass
|
Borosilicate glass
|
Soda-lime glass
|
Neutral glass
|
|
| Chemical water resistance (according to the Standard ISO 719) |
HGB 1
|
HGB 3
|
HGB 3
|
| Chemical acid resistance (according to the Standard ISO 1776) |
1
|
-
|
1
|
| Chemical alkali resistance (according to the Standard ISO 695) |
2
|
-
|
A-2
|
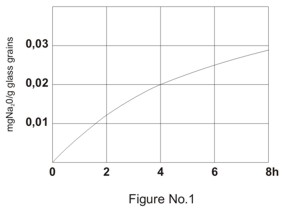
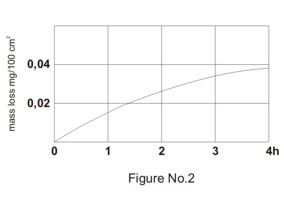
The impact of acids and water on the surface of borosilicate glass is insignificant. Only a very small amount of monovalent ions is released into the surrounding medium at the beginning of the impact. Non-porous layer of silica gel is created gradually on the glass surface. The layer works as an inhibitor against the further influence of the surrounding medium. Hydrofluoric acid and hot phosphoric acid are the only exceptions from the above-described mechanism of impacts of water and acids on the glass surface. Both acids prevent the creation of silica gel layer on the glass surface. Typical time dependence of sodium oxide loss from the glass surface by the impact of water and mass loss of the glass by the impact of acids related to cm2 is shown in Figures No.1 and 2.
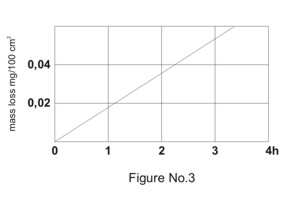
Alkali resistance
Impact of alkalies on the glass surface is increasing with alkali concentration and with temperature of the medium. In case of borosilicate glass the erosion is limited on few micrometers of the surface layer but the surface etching can remove the scales from the volumetric laboratory glass in this type of medium. Illustration of time dependence of mass loss of the glass in alkali environment is shown in Figure No. 3.
 |
 |
 |

